General research interest:
Many undesired side-effects or therapeutic failure of drugs are the results of differences or alterations of drug metabolism. Our team deals with interindividual differences in drug metabolism and elimination for more than 20 years. Our research activity focuses on the function and regulation of cytochrome P450 (CYP) enzymes, primarily involved in the metabolism of xenobiotics.
Biochemical, molecular biological and mass spectrometric approaches are applied for studying I) metabolism and pharmacokinetic interactions of drugs and drug-candidates under development, II) factors influencing the expression and function of CYP enzymes (hormonal status, disease, drug therapy), III) moreover, diagnostic approaches for patients’ drug metabolism capacity provide tools for personalized medication.
Main research projects:
1. Genetic and non-genetic factors influencing the activity of CYP enzymes
Pharmacogenetic analysis of CYP enzymes involved in drug metabolism contributes to personalized drug therapy. Phenotypes of drug-metabolizing enzymes are primarily influenced by genetic polymorphisms (loss-of-function or gain-of-function mutations, copy number variations) resulting in decreased or increased enzyme activity or complete loss of enzyme function. However, non-genetic factors (e.g. age, sex, enzyme inhibition and induction caused by drug interactions as well as diseases) can transiently decrease or increase metabolic activity leading to the phenotype different from that predicted from genotype. This phenoconversion can substantially modify the genotype based estimation of the activities of drug-metabolizing enzymes.

Role of genetic and non-genetic factors in the development of individual drug metabolism capacity.
The effects of clinically relevant polymorphic CYP allele-variants were investigated using liver tissues of human organ donors. Gene specific and accurate methods for genotyping and haplotype determination are crucial in estimation of the impact of polymorphic CYP alleles that required development of PCR-based methods for identification of CYP1A2, CYP2B6 and CYP2D6 variants. Besides genetic polymorphisms, several non-genetic factors including sex, drug therapies, chronic alcohol consumption were proved to significantly alter the phenotype predicted from CYP genotypes, for example in case of CYP2B6, CYP2C9, CYP2C19, CYP2D6 enzymes.

Hepatic CYP2C9 activity (tolbutamide 4′-hydroxylation) in subjects carrying various CYP2C9 genotypes. The activity predicted from CYP2C9 genotype was modified by non-genetic factors (CYP2C9 inducer and inhibitor therapy, amoxicillin + clavulanic acid treatment, chronic alcohol consumption), while it was not affected by the CYP2C8 genotype. The median CYP2C9 activity (dotted line) is for the cutoff value between high and low intermediate metabolizers. PM poor metabolizer, IM intermediate metabolizer, EM extensive metabolizer. * P < 0.05; ** P < 0.001
Modelling of cross-talk between sterol homeostasis and drug metabolism
The natural changes in hormonal status, or therapeutic steroids can cause substantial alteration in CYP gene expression and in CYP enzyme protein levels. The cross-talk between drug-metabolizing CYP enzymes and steroids such as dehydroepiandrosterone (DHEA), dexamethasone and cholesterol is investigated.

Dehydroepiandrosterone activates the hepatic CAR (constitutive androstane receptor) nuclear receptor, which enters the nucleus and induces the transcriptions of CYP2B6, CYP2C9, CYP2C19 and CYP3A4 genes.
2. Strategy for personalized medication adjusted to patients’ drug-metabolizing capacity
CYPtestTM, the multi-step diagnostic system for the estimation of patients’ drug-metabolizing capacity, identifies defective CYP alleles by DNA analysis (CYP-genotyping) and provides information about the current expression of key drug-metabolizing CYP enzymes by CYP-phenotyping. CYPtestTM has been introduced for patients who are on multi-drug therapy or for those who particularly benefit from tailored medication by increasing drug efficacy and by significantly decreasing the risk of the toxicity. The main focus is on the patients’ reduced or even extensive drug-metabolizing capacity (transplant recipients, psychiatric and neurologic patients as well as in patients suffering from liver dysfunction and cardiovascular diseases) which may potentially lead to therapeutic failure and severe adverse drug reactions. By recognizing poor or extensive drug metabolism, tailored medication adjusted to the patients’ drug-metabolizing capacity (by the optimization of the most appropriate drug and dosage) can minimize harmful side effects and ensure a more rational drug therapy and a more successful outcome.
Possibilities of personalized drug therapy
Systematic evaluation of the role of drug-metabolizing CYP enzymes in the metabolism of the antipsychotics, mood-stabilizers and antiepileptics frequently applied in treatment of psychiatric and neurologic patients as well as of immunosuppressant drugs, the main components of transplant patients’ therapy. The contribution of CYP polymorphisms (genetic and non-genetic variations) to inter-individual differences in the efficacy and toxicity of these drugs are estimated.
2.1. CYP3A-status, taking both CYP3A4 expression and CYP3A5 genotype into account, influences recipients’ calcineurin inhibitor therapy after transplantation. In liver transplant patients, CYP3A-status of the donor liver contributes to the recipient’s blood concentrations of ciclosporin and tacrolimus. It has been reported that patients transplanted with liver grafts from low or high expressers or with grafts carrying functional CYP3A5*1 allele required substantial modification of the initial calcineurin inhibitor dose. Donor livers’ CYP3A-status can better identify the risk of calcineurin inhibitor over- or underexposure, and may contribute to the avoidance of misdosing-induced graft injury in the early postoperative period.
The time for achieving therapeutic tacrolimus concentration was significantly reduced, confirming potential benefit of initial tacrolimus therapy adjusted to donor’s CYP3A-status over classical clinical practice of tacrolimus concentration guided treatment (4 vs 8 days, P<0.0001). Acute rejection episodes (3.6% vs 23.8%, P<0.0001) and tacrolimus induced nephrotoxicity (8% vs 27%, P=0.0004) were less frequent in patients on CYP3A-status guided tacrolimus therapy.
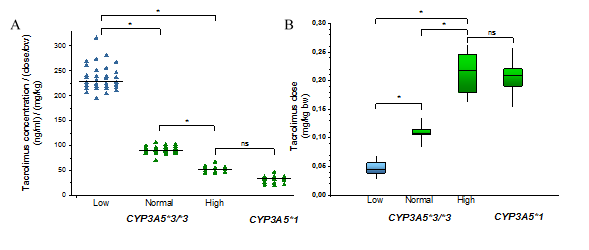
A) Influence of the donors’ CYP3A status (CYP3A5 genotypes and CYP3A4 expression) on the blood concentrations of tacrolimus in liver transplant patients.
B) Dose requirements of recipients in the course of CYP3A status of the liver donors are presented. (Low, Normal, High: the levels of CYP3A4 expression; ns: not statistically significant)
Previous experience in liver transplant patients contributed to personalized immunosuppressive therapy in heart transplant patients. In the early postoperative immunosuppressive therapy, high-dose corticosteroid treatment affected drug metabolizing capacity of patients by regulating transcription of CYP3A enzymes. In the 15-month post-operative period, The research group investigated the alterations in heart transplant recipient’s drug metabolizing capacity and phenoconverting factors that modified pharmacokinetics of immunosuppressive agents (primarily tacrolimus).

The dose-corrected tacrolimus blood concentrations in heart transplant recipients with CYP3A5*3/*3 genotype (A) and recipients carrying CYP3A5*1 allele (B) in a 15-month period after transplantation. The dotted line represents the daily dose of corticosteroid. *P<0.05, **P<0.001, ***P<0.0001
2.2. The clinical consequences of decreased CYP2C9 function were investigated in epileptic children. It has been established that valproic acid, one of the first choices of antiepileptic drugs, is metabolized primarily by CYP2C9 in pediatric patients. Identification of loss-of-function mutations in CYP2C9 may lead to false prediction of a patient’s valproate metabolizing capacity, since CYP2C9 expression highly influences blood concentrations of valproate.

Serum concentrations of valproic acid in epileptic children with different CYP2C9-statuses. (CYP2C9*1/mut: heterozygous CYP2C9 genotype (CYP2C9*1/*2 or CYP2C9*1/*3); normal: intermediate CYP2C9 expressions; Low: low CYP2C9 expressions; *P <0.0001)
Patients’ CYP2C9-status guided dosing strategy for achieving the optimal blood concentration has been suggested.

Valproic acid dose required for the therapeutic serum concentrations epileptic children with various CYP2C9-statuses.
CYP2C9-guided (CYP2C9 genotype and CYP2C9 expression) treatment significantly reduced the ratio of patients out of the range of target valproate blood concentrations, the ratio of patients with abnormal serum alkaline phosphatase levels and the incidence of serious side effects, notably hyperammonemia.
2.3. The incidence of adverse reactions in the anticonvulsant clonazepam therapy is highly attributed to the inter-individual variability in clonazepam metabolism by CYP3A and NAT2 (N-acetyl transferase 2) enzymes. The patients’ CYP3A4 expression was found to be the major determinant of clonazepam plasma concentrations; whereas CYP3A5 genotype and NAT2 acetylator phenotype did not influence the steady state levels of clonazepam.

A) Clonazepam concentrations normalized by dose and bodyweight in the patients expressing CYP3A4 at low, normal, and high levels. Yellow points indicate patients carrying CYP3A5*1
B)7-aminoclonazepam/clonazepam concentrations in patients with various CYP3A4 expression and NAT2 acetylator phenotype, *P <0.0001
However, the normal CYP3A4 expression and slow NAT2 acetylation phenotype evoking high plasma concentration ratio of 7-amino-clonazepam and clonazepam, may account for low efficacy or withdrawal symptoms of clonazepam. Prospective assaying of CYP3A4 expression and NAT2 acetylation phenotype can better identify the patients with higher risk of adverse reactions and can facilitate the improvement of personalized clonazepam therapy and withdrawal regimen.
2.4. The atypical antipsychotic clozapine is effective in treatment-resistant schizophrenia; however, the success or failure of clozapine therapy is substantially affected by the variables that impact the clozapine blood concentration. CYP3A4 expression was found to be the major determinant of normalized clozapine concentration, particularly in patients expressing CYP1A2 at relatively low level. The functional CYP3A5*1 allele seemed to influence clozapine concentrations in those patients who expressed CYP3A4 at low levels. Strong association was observed between the metabolite/clozapine ratios and CYP3A4 mRNA levels, which confirmed the primary role of CYP3A4 in clozapine metabolism.
3. Development of in vitro models
Various in vitro models have been developed for in vitro testing of drugs that are useful in pharmacological, pharmacokinetic, and toxicity studies.
3.1. Human induced pluripotent stem cell-derived brain organoids in pharmacology and toxicology studies
Aging societies face new challenges, one of the most serious being the increasing incidence of cancer and neurodegenerative diseases. In order to successfully combat these diseases, researchers are working to map the human body as accurately as possible when developing model systems. In the case of the brain, cerebral organoids are able to do this, properly reproducing not only the cell types that make up the cortical plate, but also their structural arrangement. From these organoids, slice cultures are created that can be sustained for up to one year when grown at the air-liquid interface. Thus, mature neuronal and glia cell types can develop that allow the study of specific pathological changes in neurodegenerative diseases such as amyotrophic lateral sclerosis with frontotemporal dementia (ALS / FTD) and screening of drug molecules that can potentialy prevent or slow down the disease (Szebényi et al., 2021).
3.2. Pharmacokinetic variations leading to therapy resistence in cancer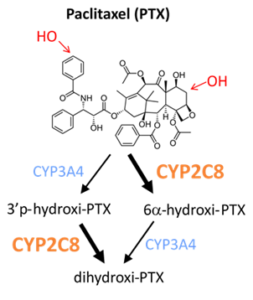
The research group in collaboration with National Korányi Institute of Pulmonology and Drug Resistance Research Group investigates the role of pharmacokinetic variability in drug resistance that highly impact clinical outcome of cancer therapy. The major aim is to adapt and further develop in vitro modell systems to reveal resistance mechanisms against the anticancer agent paclitaxel (adenocarcinoma cell lines overexpressing CYPs).
3.3. Development of biomarkers for toxicological and safety studies
One of the main goals of the research team’s work is to identify extracellular microRNA-based biomarkers that are able to detect organ-specific damage earlier than conventional methods. miRNAs are RNA molecules that do not encode a protein but can bind to the 3 ‘untranslated region of mRNAs and inhibit its transcription as well as induce its degradation. A functionally determined proportion of miRNAs are found in extracellular vesicles. Validation of novel biomarker candidates is performed in vitro in rat cell model and in vivo in laboratory animal model.
3.4. In vitro model for testung immunosuppression efficacy
Ex vivo immunostimulation model has been developed using peripheral blood mononuclear cells to characterize immunosuppressive agents on the basis of their inhibitory effects on the expression of pro-inflammatory cytokines. The model is to be used to assaying the efficacy of immunosuppressive therapy after organ transplantation and the immunological background of acute rejection events.
Lab Equipments:
- Cell-culture laboratory equipped with CO2 incubator, Esco Class II Biological Safety Cabinet, microscope
- HPLC UV-VIS, radiodetector
- Fluorimeter
- NanoDrop 1000 Spectrophotometer
- Real-time PCR and end-point PCR systems (BioRad)
- Western blot systems
- Fluidigm BiomarkTM HD high throughput PCR system
- Bio-Rad Droplet Digital PCR systems
- QuantStudio 5 real-time PCR systems
- Agilent 4200 TapeStation nucleic acid analyzer
- Ultracentrifuge
Collaborations:
- Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia – The cross-talk between cholesterol homeostasis and drug- metabolism
- Palacky University, Olomouc, Czech Republic – The effect of the steroid type compounds (dexamethasone, dehydroepiandrosterone) on drug-metabolizing cytochrome P450 enzymes
- INSERM (Institut National de la Santé et de la Recherche Médicale) U632, Montpellier, France – The role of nuclear receptors in the regulation of cytochrome P450 enzymes
- University of Cambridge, Department of Clinical Neurosciences, Lakatos Lab – Development of ALS/FTD organoid models
- Department of Transplantation and Surgery, Semmelweis University, Budapest – Drug metabolism in transplant patients; Prevention of toxicity resulted from ciclosporin metabolism
- Heart and Vascular Center, Semmelweis University, Budapest – Drug metabolism in transplant patients; Prevention of toxicity resulted from immunosuppressant metabolism
- Department of Psychiatry and Psychotherapy, Semmelweis University, – Possibilities of personalized antipsychotic therapy
- Madarász Hospital, Heim Pál Children’s Hospital , Budapest – Personalized antiepileptic therapy
- 2nd Department of Pediatrics, Semmelweis University – CYP copy number variations in tumorous tissues
- St László Hospital, Budapest – Personalized therapy of bone-marrow transplant patients
- 1st Department of Surgery, Semmelweis University, Budapest – The effect of portal vein ligation on drug-metabolizing function of the liver
- National Korányi Institute of Pulmonology – The mechanisms of therapy resistance, designing personalized therapies
- Centre for Energy Research, Institute of Technical Physics and Materials Science, Microsystems Laboratory – Establishing physiological organoid culture technology
Education/training:
- Semmelweis University, Budapest
- Eötvös Loránd University, Budapest
- Budapest University of Technology and Economics, Budapest
Selected publications:
Szebényi K, Wenger LMD, Sun Y, Dunn AWE, Limegrover CA, Gibbons GM, Conci E, Paulsen O, Mierau SB, Balmus G and Lakatos A: Human ALS/FTD Brain Organoid Slice Cultures Display Distinct Early Astrocyte and Targetable Neuronal Pathology. Nature Neuroscience 24: 1542–1554, 2021
Déri M, Szakál-Tóth Zs, Fekete F, Mangó K, Incze E, Minus A, Merkely B, Sax B, Monostory K: CYP3A-status is associated with blood concentration and dose-requirement of tacrolimus in heart transplant recipients Scientific Reports 11: 21389, 2021
Fekete F, Mangó K, Déri M, Incze E, Minus A, Monostory K: Impact of genetic and non-genetic factors on hepatic CYP2C9 expression and activity in Hungarian subjects. Scientific Reports 11: 17081, 2021
Csikány N, Kiss Á, Déri M, Fekete F, Minus A, Tóth K, Temesvári M, Sárváry E, Bihari L, Gerlei Zs, Kóbori L, Monostory K: Clinical significance of personalized tacrolimus dosing by adjusting to donor CYP3A-status in liver transplant recipients. British Journal of Clinical Pharmacology 87: 1790–1800, 2021
Menus Á, Kiss Á, Tóth K, Sirok D, Déri M, Fekete F, Csukly G, és Monostory K: Association of clozapine-related metabolic disturbances with CYP3A4 expression in patients with schizophrenia. Scientific Reports 10: 212383, 2020
Déri M. T, Kiss Á. F, Tóth K, Paulik J, Sárváry E, Kóbori L, Monostory K: End-stage renal disease reduces the expression of drug-metabolizing cytochrome P450s. Pharmacological Reports 72: 1695–1705, 2020
Kiss Á, Menus Á, Tóth K, Déri M, Sirok D, Gabri E, Belic A, Csukly G, Bitter I, Monostory K: Phenoconversion of CYP2D6 by inhibitors modifies aripiprazole exposure. European Archives of Psychiatry and Clinical Neuroscience 270: 71–82, 2019
Monostory K, Nagy A, Tóth K, Bűdi T, Kiss Á, Déri M, Csukly G: Relevance of CYP2C9 function in valproate therapy. Current Neuropharmacology 17: 99–106, 2019
Kiss Á. F, Vaskó D, Déri M. T, Tóth K, Monostory K: Combination of CYP2C19 genotype with non-genetic factors evoking phenoconversion improves phenotype prediction. Pharmacological Reports 70: 525–532, 2018
Kiss Á, Tóth K, Juhász C, Temesvári M, Paulik J, Hirka G, Monostory K: Is CYP2D6 phenotype predictable from CYP2D6 genotype? Microchemical Journal 136: 209-214, 2018
Tóth K, Csukly G, Sirok D, Belic A, Kiss Á, Háfra E, Déri M, Menus Á, Bitter I, Monostory K: Potential role of patients’ CYP3A-status in clozapine pharmacokinetics. International Journal of Neuropsychopharmacology 20: 529-537, 2017
Tóth K, Csukly G, Sirok D, Belic A, Háfra E, Kiss Á, Déri M, Menus Á, Bitter I, Monostory K: Optimization of clonazepam therapy adjusted to patient’s CYP3A-status and NAT2 genotype. International Journal of Neuropsychopharmacology 19: 1-9, 2016
Bűdi T, Tóth K, Nagy A, Szever Z, Kiss Á, Temesvári M, Háfra E, Garami M, Tapodi A, Monostory K: Clinical significance of CYP2C9-status guided valproic acid therapy in children. Epilepsia 56: 849-855, 2015
Monostory K, Tóth K, Kiss Á, Háfra E, Csikány N, Paulik J, Sárváry E, Kóbori L: Personalizing calcineurin inhibitor therapy by adjusting to donor CYP3A-status in liver transplant patients British Journal of Clinical Pharmacology 80: 1429-1437, 2015
Tóth K, Bűdi T, Kiss Á, Temesvári M, Háfra E, Nagy A, Szever Z, Monostory K: Phenoconversion of CYP2C9 in epilepsy limits the predictive value of CYP2C9 genotype in optimizing valproate therapy. Personalized Medicine 12: 199-207, 2015
Szebényi K, Péntek A, Erdei Z, Várady G, Orbán TI, Sarkadi B, Apáti Á: Efficient generation of human embryonic stem cell-derived cardiac progenitors based on tissue-specific enhanced green fluorescence protein expression. Tissue Engineering, Part C: Methods 21: 35-45, 2015
Szebényi K, Füredi A, Kolacsek O, Csohány R, Prókai Á, Kis-Petik K, Szabó A, Bősze Z, Bender B, Tóvári J, Enyedi Á, Orbán TI, Apáti Á, Sarkadi B: Visualization of Calcium Dynamics in Kidney Proximal Tubules. Journal of the American Society of Nephrology 26: 2731-2740, 2015
Szebényi K, Füredi A, Kolacsek O, Pergel E, Bősze Z, Bender B, Vajdovich P, Tóvári J, Homolya L, Szakács G, Héja L, Enyedi Á, Sarkadi B, Apáti Á, Orbán TI: Generation of a Homozygous Transgenic Rat Strain Stably Expressing a Calcium Sensor Protein for Direct Examination of Calcium Signaling. Scientific Reports 5: 12645, 2015
Belic A, Tóth K, Vrzal R, Temesvári M, Porrogi P, Orbán E, Rozman D, Dvorak Z, Monostory K: Dehydroepiandrosterone post-transcriptionally modifies CYP1A2 induction involving androgen receptor. Chemico-Biological Interactions 203: 597-603, 2013
Temesvári M, Kóbori L, Paulik J, Sárváry E, Belic A, Monostory K: Estimation of drug-metabolizing capacity by cytochrome P450 genotyping and expression. Journal of Pharmacology and Experimental Therapeutics 341: 294-305, 2012
Temesvári M, Paulik J, Kóbori L, Monostory K: High-resolution melting curve analysis to establish CYP2C19*2 single nucleotide polymorphism: comparison with hydrolysis SNP analysis. Molecular and Cellular Probes 25: 130-133, 2011
Monostory K, Dvorak Z: Steroid regulation of drug-metabolizing cytochromes P450. Current Drug Metabolism 12: 154-172, 2011
Rezen T, Rozman D, Pascussi J-M, Monostory K: Interplay between cholesterol and drug metabolism. Biochim Biophys Acta – Proteins and Proteomics 1814: 146-160, 2011
Rozman D, Monostory K: Perspectives of the non-statin hypolipidemic agents. Pharmacology and Therapeutics 127: 19-40, 2010
Belic A, Temesvári M, Kőhalmy K, Vrzal R, Dvorak Z, Rozman D, Monostory K: Investigation of the CYP2C9 induction profile in human hepatocytes by combining experimental and modelling approaches. Current Drug Metabolism 10: 457-461, 2009
Monostory K, Pascussi J-M, Kóbori L, Dvorak Z: Hormonal regulation of CYP1A expression. Drug Metabolism Reviews 41: 547-572, 2009
Monostory K, Pascussi J-M, Szabó P, Temesvári M, Kőhalmy K, Acimovic J, Kocjan D, Kuzman D, Wilzewski B, Bernhardt R, Kóbori L, Rozman D: Drug-interaction potential of 2-((3,4-(dichlorophenethyl(propyl)amino)-1-(pyridin-3-yl)ethanol (LK-935), the novel non-statin type cholesterol lowering agent. Drug Metabolism and Disposition 37: 375-385, 2009
Kóbori L, Kőhalmy K, Porrogi P, Sárváry E, Gerlei Zs, Fazakas J, Nagy P, Járay J, Monostory K: Drug-induced liver graft toxicity caused by cytochrome P450 poor metabolism. British Journal of Clinical Pharmacology 65: 428-436, 2008
Kőhalmy K, Tamási V, Kóbori L, Sárváry E, Pascussi J-M, Porrogi P, Rozman D, Prough RA, Meyer UA, Monostory K: Dehydroepiandrosterone induces human CYP2B6 through the constitutive androstane receptor. Drug Metabolism and Disposition 35: 1495-1501, 2007
Monostory K, Kőhalmy K, Prough, RA, Kóbori L, Vereczkey L: The effect of synthetic glucocorticoid, dexamethasone on CYP1A1 inducibility in adult rat and human hepatocytes. FEBS Letters 579: 229-235, 2005
Monostory K, Hazai E, Vereczkey L: Inhibition of cytochrome P450 enzymes participating in p-nitrophenol hydroxylation by drugs known as CYP2E1 inhibitors. Chemico-Biological Interactions 147: 331-340, 2004
Szűcs G, Tamási V, Laczay P, Monostory K: Biochemical background of toxic interaction between tiamulin and monensin. Chemico-Biological Interactions 147: 151-161, 2004
Tamási V, Hazai E, Porsmyr-Palmertz M, Ingelman-Sundberg M, Vereczkey L, Monostory K: GYKI-47261, a new AMPA antagonist is a CYP2E1 inducer. Drug Metabolism and Disposition 31:1310-1314, 2003
Tamási V, Vereczkey L, Falus A, Monostory K: Some aspects of interindividual variations in the metabolism of xenobiotics. Inflammation Research 52:322-333, 2003
Hazai E, Vereczkey L, Monostory K: Reduction of toxic metabolite formation of acetaminophen. Biochemical Biophysical Research Communications 291: 1089-1094, 2002
Tamási V, Kiss Á, Dobozy O, Falus A, Vereczkey L, Monostory K: The effect of dexamethasone on P450 activities in regenerating liver. Biochemical Biophysical Research Communications 286: 239-242, 2001
Monostory K, Vereczkey L, Lévai F, Szatmári I: Iprifalvone as an inhibitor of human cytochrome P450 enzymes. British Journal of Pharmacology 123: 605-610, 1998
Monostory K, Jemnitz K, Vereczkey L, Czira G: Species differences in metabolism of panomifene, an analogue to tamoxifen. Drug Metabolism and Disposition 25: 1370-1378, 1997
Monostory K, Vereczkey L: The effect of phenobarbital and dexamethasone coadministration on the activity of rat liver P450 system. Biochemical Biophysical Research Commununications 203: 351-358, 1994
Leader
Katalin Monostory











