Research Overview
The conformational diseases such as Parkinson’s disease (PD) and Multiple System Atrophy (MSA) address a significant gathering of neurodegenerative issues. The signs of these illnesses are the α-synuclein (SYN) and the as of late found Tubulin Polymerization Promoting Protein (TPPP/p25) by the CellArchitecture team. The two proteins with chameleon characteristics express their functions separately in neurons and oligodendrocytes (OLGs); despite this fact, they are co-enriched and co-limited in obsessive incorporations on account of PD and MSA. The hallmarks signs of Parkinson disease (PD) and other synucleinopathies, Tubulin Polymerization Promoting Protein (TPPP/p25) and α-synuclein (SYN) have two key highlights: they are cluttered and co-accumulated in inclusions (Oláh et al., 2020). These Neomorphic Moonlighting Proteins show both physiological and neurotic capacities due to their intrinsically unstructured features leading distinct heteroassociations. To achieve the particular focusing of the neurotic TPPP/p25-SYN; however, not the physiological TPPP/p25-tubulin complex, their interfaces were recognized as a particular inventive procedure for the improvement of anti-Parkinson drugs (Oláh et al., 2021).
In the light of these issues, we established a new innovative strategy for the validation of a specific drug target based upon the identification of contact surfaces of the pathological SYN-TPPP/p25 complex that may lead to the development of peptidomimetic foldamers suitable for pharmaceutical intervention (Szunyogh et al., 2015; Szénási et al., 2017). In addition, the autophagy degradation of the accumulated/assembled SYN has been considered as a potential therapeutic target. We have shown that the hetero-association of SYN with TPPP/p25 after their uptake from the medium by human cells (which mimics cell-to-cell transmission) inhibits both their autophagy- and the ubiquitin-proteasome system-derived elimination (cf. introductory picture) (Lehotzky et al., 2021). The combined strategy may ensure efficient elimination of the pathological SYN level.
At physiological conditions, TPPP/p25 regulates the elements and strength of the microtubule framework; its demeanor is significant for the OLGs, the key constituents of the myelin sheath. The get together of TPPP/p25 and SYN, as deadly drive, of the etiology of PD and MSA has been set up. Because of the remarkable underlying and useful highlights of TPPP/p25, another inventive procedure must be assessed to repress as well as destruct explicitly the association of TPPP/p25 with SYN; this could be satisfied by focusing on the interface of the obsessive complex without influencing the physiological one. In addition, our examinations underline that focusing on multifunctional proteins is a difficult undertaking; by and by, the approval of a medication target can be accomplished by distinguishing the interface of edifices of the accomplice proteins existing at the given pathological conditions.
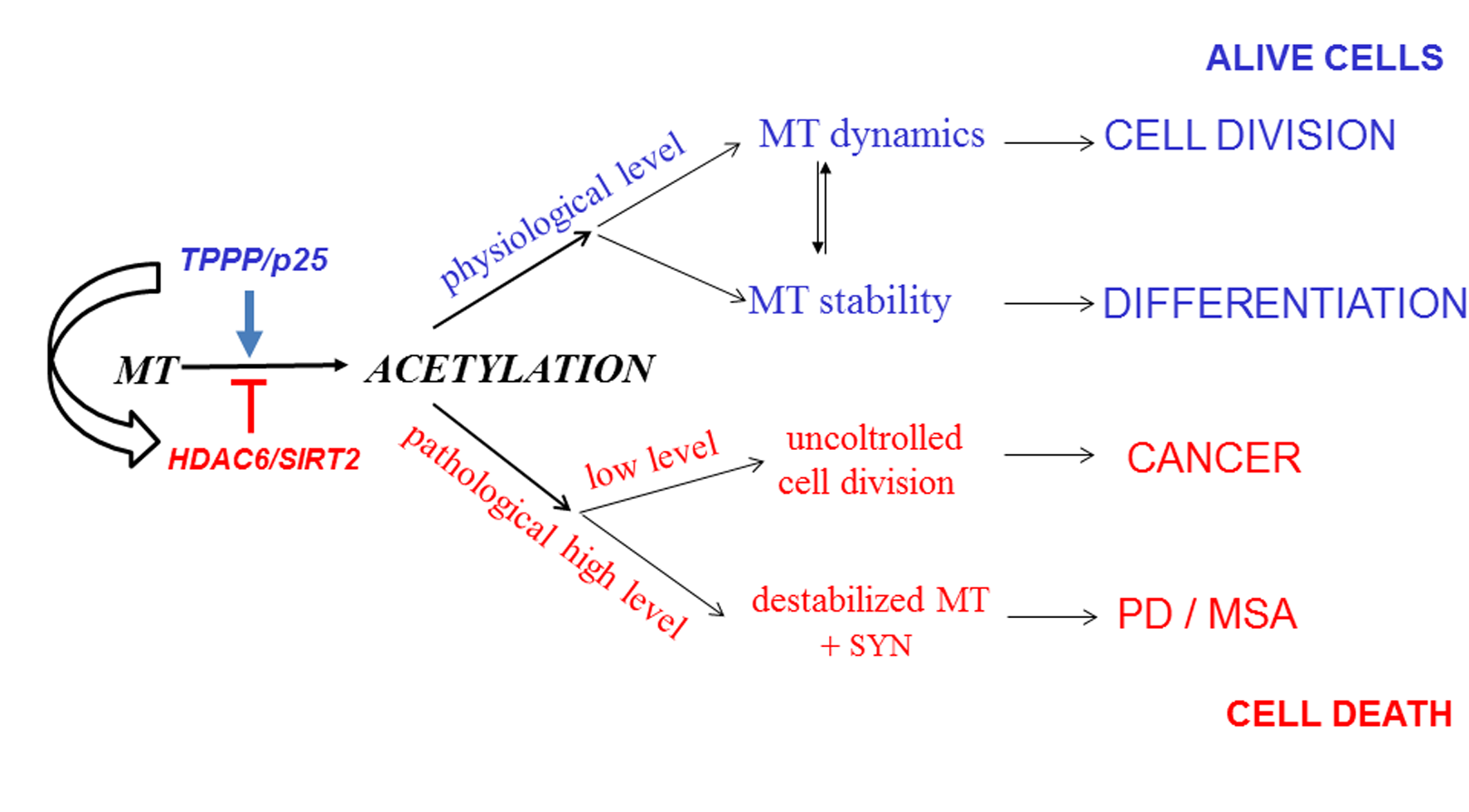 Truth to be told, the investigations of the different associations at sub-atomic and cell levels uncovered the high conformational versatility, chameleon include, of TPPP/p25 that guarantees outstanding useful interacting potency. Physiologically, the moonlighting TPPP/p25 modulates the dynamics and stability of the microtubule network by bundling microtubules and enhancing the tubulin acetylation due to the inhibition of tubulin deacetylases (Szabó et al., 2017; Schiedel et al., 2018 and 2020). The optimal endogenous TPPP/p25 level is crucial for its physiological functions. TPPP/p25-derived microtubule hyperacetylation counteracts uncontrolled cell division. According to a recent recognition, cytoskeletal filaments play a role in SARS-CoV-2 virus infection, the COVID-19 pandemic (Norris and Ovádi, 2021).All these issues reveal the anti-mitotic and alpha-synuclein aggregation-promoting potency of TPPP/p25, consistent with the finding that PD patients have reduced risk for certain cancers.
Truth to be told, the investigations of the different associations at sub-atomic and cell levels uncovered the high conformational versatility, chameleon include, of TPPP/p25 that guarantees outstanding useful interacting potency. Physiologically, the moonlighting TPPP/p25 modulates the dynamics and stability of the microtubule network by bundling microtubules and enhancing the tubulin acetylation due to the inhibition of tubulin deacetylases (Szabó et al., 2017; Schiedel et al., 2018 and 2020). The optimal endogenous TPPP/p25 level is crucial for its physiological functions. TPPP/p25-derived microtubule hyperacetylation counteracts uncontrolled cell division. According to a recent recognition, cytoskeletal filaments play a role in SARS-CoV-2 virus infection, the COVID-19 pandemic (Norris and Ovádi, 2021).All these issues reveal the anti-mitotic and alpha-synuclein aggregation-promoting potency of TPPP/p25, consistent with the finding that PD patients have reduced risk for certain cancers.
References
Lehotzky A, Oláh J, Fekete JT, Szénási T, Szabó E, Győrffy B, Várady G, Ovádi J. Co-Transmission of Alpha-Synuclein and TPPP/p25 Inhibits Their Proteolytic Degradation in Human Cell Models. Front Mol Biosci. 2021 May 18;8:666026. doi: 10.3389/fmolb.2021.666026. eCollection 2021. PMID: 34084775
Norris V, Ovádi J. Role of Multifunctional Cytoskeletal Filaments in Coronaviridae Infections: Therapeutic Opportunities for COVID-19 in a Nutshell. Cells. 2021 Jul 19;10(7):1818. doi: 10.3390/cells10071818. PMID: 34359986
Oláh J, Lehotzky A, Szunyogh S, Szénási T, Orosz F, Ovádi J. Microtubule-Associated Proteins with Regulatory Functions by Day and Pathological Potency at Night. Cells. 2020 Feb 4;9(2):357. doi: 10.3390/cells9020357. PMID: 32033023
Oláh J, Lehotzky A, Szénási T, Ovádi J. A potential innovative therapy for Parkinson’s disease: Selective destruction of the pathological assemblies of alpha-synuclein. In: Lin, Zhang (szerk.) Dementia in Parkinson’s Disease. London, Egyesült Királyság / Anglia : IntechOpen (2021)
Schiedel M, Lehotzky A, Szunyogh S, Oláh J, Hammelmann S, Wössner N, Robaa D, Einsle O, Sippl W, Ovádi J, Jung M. HaloTag-Targeted Sirtuin-Rearranging Ligand (SirReal) for the Development of Proteolysis-Targeting Chimeras (PROTACs) against the Lysine Deacetylase Sirtuin 2 (Sirt2)*. Chembiochem. 2020 Dec 1;21(23):3371-3376. doi: 10.1002/cbic.202000351. Epub 2020 Aug 27. PMID: 32672888
Schiedel M, Herp D, Hammelmann S, Swyter S, Lehotzky A, Robaa D, Oláh J, Ovádi J, Sippl W, Jung M. Chemically Induced Degradation of Sirtuin 2 (Sirt2) by a Proteolysis Targeting Chimera (PROTAC) Based on Sirtuin Rearranging Ligands (SirReals). J Med Chem. 2018 Jan 25;61(2):482-491. doi: 10.1021/acs.jmedchem.6b01872. Epub 2017 Apr 17. PMID: 28379698
Szabó A, Oláh J, Szunyogh S, Lehotzky A, Szénási T, Csaplár M, Schiedel M, Lőw P, Jung M, Ovádi J. Modulation Of Microtubule Acetylation By The Interplay Of TPPP/p25, SIRT2 And New Anticancer Agents With Anti-SIRT2 Potency. Sci Rep. 2017 Dec 6;7(1):17070. doi: 10.1038/s41598-017-17381-3. PMID: 29213065
Szénási T, Oláh J, Szabó A, Szunyogh S, Láng A, Perczel A, Lehotzky A, Uversky VN, Ovádi J. Challenging drug target for Parkinson’s disease: Pathological complex of the chameleon TPPP/p25 and alpha-synuclein proteins. Biochim Biophys Acta Mol Basis Dis. 2017 Jan;1863(1):310-323. doi: 10.1016/j.bbadis.2016.09.017. Epub 2016 Sep 24. PMID: 27671864
Szunyogh S, Oláh J, Szénási T, Szabó A, Ovádi J. Targeting the interface of the pathological complex of α-synuclein and TPPP/p25. Biochim Biophys Acta. 2015 Dec;1852(12):2653-61. doi: 10.1016/j.bbadis.2015.09.012. Epub 2015 Sep 25. PMID: 26407520
Ovádi Judit https://m2.mtmt.hu/gui2/?type=authors&mode=browse&sel=10000292
Orosz Ferenc https://m2.mtmt.hu/gui2/?type=authors&mode=browse&sel=authors10000310
Granted projects
Physiological and pathological interactions of the disordered TPPP/p25 protein: mapping of binding domains. OTKA K-101039 (2012-2016) Project coordinator: Judit Ovádi
Involvement of Tubulin Polymerization Promoting Protein (TPPP/p25) in neuroprotection achieved by autophagy, proteasome and aggresome systems. OTKA K-112144 (2015-2017) Project coordinator: Judit Ovádi
Way to the personalized therapies of the synucleinopathies: drug target validation. RICHTER Nyrt-KK/342/2018 RICHTER GEDEON Nyrt. Project coordinator: Judit Ovádi
Zinc-Net: the Network for the Biology of Zinc. EU COST Action TD1304 (2013-2017)
Epigenetic Chemical Biology (EPICHEM). EU COST Action -TD0905 (2014-2018)
Educational activity
MSc and post-graduate educations: 2 MSc, 10 PhD, 12 diploma thesis/TDK issues; International educations in Cordoba (Argentina) 2011; Araraquara (Brazilia) 2012; within the frame of EU-Latin-America projects
External committee memberships: ELTE Biological Doctoral Committee; ELTE TTK Habilitation Committee; Scientific Committee for the History of Science and Technology of the Hungarian Academy of Sciences
Collaborations
International collaborators
Prof. Marival Bermejo, Institute of Pharmacology, Miguel Hernández University, Spain
Prof. Manfed Jung, Albert-Ludwigs-Universität Freiburg, Germany
Prof. Gabor G. Kovacs, University of Toronto, Toronto, Canada
Dr. Katalin Medzihradszky, UCSF, School of Pharmacy, Department of Pharmaceutical Chemistry, San Francisco, CA, USA
Prof. Victor Norris, Faculty of Science, University of Rouen, France
Dr. László Tirián, Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA), Vienna BioCenter (VBC), Vienna, Austria
National collaborators
Prof. Timea Berki, Department of Immunology and Biotechnology, Medical School, University of Pécs, Pécs, Hungary
Prof. Anna Erdei , Department of Immunology, Eötvös Loránd University, Budapest, Hungary
Prof. Judit Fidy, Department of Biophysics and Radiation Biology, Semmelweis University, Budapest, Hungary
Prof. János Kovács , Department of Anatomy, Cell and Developmental Biology, Institute of Biology Eötvös Loránd University , Budapest, Hungary
Prof. Anna Magyar, MTA-ELTE Research Group of Peptide Chemistry, Hungarian Academy of Sciences, Eötvös Loránd University, Budapest, Hungary
Prof. Botond Penke, Department of Medical Chemistry, University of Szeged, Szeged, Hungary
Prof. András Perczel , Laboratory of Structural Chemistry and Biology, MTA-ELTE Protein Modelling Research Group, Institute of Chemistry, Eötvös Loránd University, Budapest, Hungary
Prof. László Vécsei, Department of Neurology, Faculty of Medicine, Albert Szent-Györgyi Clinical Center, University of Szeged, Szeged, Hungary
Collaborators within the RCNS
Prof. Győrffy Balázs, Oncology Biomarker Research Group, Institute of Enzymology
Dr. Várady György, Molecular cell biology Research Group, Institute of Enzymology
Dr. Tőke Orsolya, NMR Research Laboratory, Instrumentation Center
Former members released as postdoctoral fellows
Dr. István Horváth 2010, to Chalmers University of Technology, Gothenburg, Sweden
Dr. Natália Tőkési 2012, to Membrane Protein Research Group, Institute of Enzymology, RCNS
Dr. Ágnes Zotter 2013, to Department of Biomolecular Sciences, Weizmann Institute of Science, then TargetEx Biosciences
Dr. Sándor Szunyogh 2017, to Department of Physiology, Anatomy and Genetics, Division of Medical Sciences at the University of Oxford, Oxford Parkinson’s Disease Centre
Dr. Adél Szabó 2020, to Versys Clinics, Human Reproduction Institution
Leader
László Hunyady



